Daratumumab Plus Bortezomib, Melphalan, and Prednisone Versus Bortezomib, Melphalan, and Prednisone in Patients with Transplant-Ineligible Newly Diagnosed Multiple Myeloma: Overall Survival in Alcyone
- Abstract
- Presentation
Author(s): Maria-Victoria Mateos, Michele Cavo, Joan Bladé, MD PhD, Meletios A. Dimopoulos, MD, Kenshi Suzuki, MD PhD, Andrzej Jakubowiak, MD PhD, Stefan Knop, Chantal Doyen, MD, Paulo Lucio, MD PhD, Zsolt Nagy, MD PhD, Ludek Pour, MD, Mark Cook, MBChB,PhD, Sebastian Grosicki, MD PhD, Andre H Crepaldi, MD, Anna Marina Liberati, Philip Campbell, MBBS, FRACP, FRCPA, Tatiana Shelekhova, Sung-Soo Yoon, MD PhD, Genadi Iosava, MD, Tomoaki Fujisaki, MD PhD, Mamta Garg, MD FRCP, FRCPath, Maria Krevvata, PhD, Jianping Wang, Anupa Kudva, MD, Jon Ukropec, PhD, Susan Wroblewski, PhD, Rachel Kobos, MD, Jesús San-Miguel
Disclosures: Mateos:Abbvie: Membership on an entity’s Board of Directors or advisory committees; GSK: Membership on an entity’s Board of Directors or advisory committees; EDO: Membership on an entity’s Board of Directors or advisory committees; Pharmamar: Membership on an entity’s Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Adaptive: Honoraria. Cavo:Janssen, Celgene, Amgen, Abbvie: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Celgene, Janssen, Amgen, BMS, Abbvie, Takeda: Honoraria; Janssen, Celgene: Other: Travel Accommodations; Janssen, Celgene: Speakers Bureau. Bladé:Irctures: Honoraria; Janssen, Celgene, Amgen, Takeda: Membership on an entity’s Board of Directors or advisory committees. Dimopoulos:Sanofi Oncology: Research Funding. Suzuki:Ono: Research Funding; BMS: Honoraria, Research Funding; Takeda: Honoraria; Janssen: Honoraria; Celgene: Honoraria. Jakubowiak:Janssen: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; KaryoPharm Therapeutics: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Juno: Consultancy, Honoraria; Adaptive Biotechnologies: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Sanofi: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; SkyLineDx: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees; Millennium: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees. Knop:Janssen, AMGEN, Bristol-Myers Squibb, Celgene: Consultancy, Honoraria. Lucio:Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Nagy:Abbvie: Membership on an entity’s Board of Directors or advisory committees; Roche: Membership on an entity’s Board of Directors or advisory committees; Janssen: Membership on an entity’s Board of Directors or advisory committees; Novartis: Membership on an entity’s Board of Directors or advisory committees; Takeda: Membership on an entity’s Board of Directors or advisory committees. Cook:Celgene, Jannsen-Cilag, Takeda: Honoraria, Research Funding; Amgen, Bristol-Myers Squibb, GlycoMimetics, Seattle Genetics and Sanofi: Honoraria. Liberati:Janssen: Honoraria; Bristol & Mayer: Honoraria; Celgene: Honoraria; Servier: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Takeda: Membership on an entity’s Board of Directors or advisory committees; Amgen: Membership on an entity’s Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Incyte: Consultancy. Campbell:Janssen: Honoraria, Research Funding, Speakers Bureau. Garg:Novartis, Janssen: Research Funding; Janssen, Takeda, Novartis: Other: Travel expenses; Janssen: Honoraria. Krevvata:Janssen: Employment. Wang:Janssen: Employment. Kudva:Janssen: Employment, Equity Ownership. Ukropec:Janssen: Employment, Equity Ownership. Wroblewski:Janssen: Employment, Equity Ownership. Kobos:Janssen: Employment. San-Miguel:Amgen, Bristol-Myers Squibb, Celgene, Janssen, MSD, Novartis, Roche, Sanofi, and Takeda: Consultancy, Honoraria.
Introduction: Daratumumab (DARA) is a human IgGκ monoclonal antibody targeting CD38 with a direct on-tumor and immunomodulatory mechanism of action. The addition of DARA to standard-of-care regimens in phase 3 studies reduced the risk of disease progression or death by ≥44%, nearly doubled the rate of complete response or better, and induced a ≥3-fold increase in minimal residual disease (MRD)-negativity rates versus standard of care alone in patients with transplant-ineligible newly diagnosed multiple myeloma (NDMM) and relapsed/refractory multiple myeloma. In the primary analysis of the phase 3 ALCYONE study (median follow-up: 16.5 months), a significant progression-free survival (PFS) benefit (median not reached vs 18.1 months; hazard ratio [HR], 0.50; P <0.001) was observed with the addition of DARA to bortezomib/melphalan/prednisone (D-VMP) in patients with transplant-ineligible NDMM, without an increase in overall toxicity (Mateos MV, et al. N Engl J Med. 2018;378[6]:518-528). D-VMP continued to demonstrate a significant PFS benefit versus VMP alone after 1 year of additional follow-up, including in patients ≥75 years of age (Dimopoulos MA, et al. Blood. 2018;132[Suppl 1]:156). After a median follow-up of 27.8 months, D-VMP reduced the risk of disease progression or death by 57% versus VMP alone, with a 24-month PFS rate of 63% in the D-VMP group and 36% in the VMP group. This PFS benefit was observed regardless of patient age and was maintained during the subsequent line of therapy in patients with transplant-ineligible NDMM. Here, we present >36 months of follow-up from ALCYONE, including analysis of overall survival (OS) from a prespecified interim analysis.
Methods: Patients with NDMM ineligible for high-dose chemotherapy and autologous stem cell transplantation due to age (≥65 years) or comorbidities were randomized 1:1 to receive up to nine 6-week cycles of VMP (bortezomib 1.3 mg/m2 subcutaneously on Days 1, 4, 8, 11, 22, 25, 29, and 32 of Cycle 1 and Days 1, 8, 22, and 29 of Cycles 2-9; melphalan 9 mg/m2 orally and prednisone 60 mg/m2 orally on Days 1-4 of Cycles 1-9) with or without DARA (16 mg/kg intravenously once weekly for Cycle 1, once every 3 weeks for Cycles 2-9, and once every 4 weeks for Cycles 10+ until disease progression). The primary endpoint was PFS. Secondary endpoints included overall response rate, rate of complete response or better, rate of very good partial response or better, MRD-negativity rate (10-5 threshold), PFS on subsequent line of therapy (PFS2), OS, and safety.
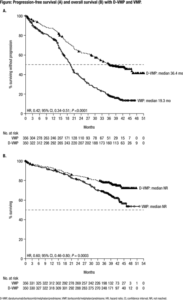
Results: A total of 706 patients were enrolled in this study (D-VMP: n = 350; VMP: n = 356). Patient baseline characteristics were well balanced between treatment arms. The median (range) age was 71 (40-93) years, and 29.9% of patients were ≥75 years of age. 518 (84.1%) and 98 (15.9%) of 616 patients evaluated had standard and high (del17p, t[14;16], and/or t[4;14] positive) cytogenetic risk, respectively, as assessed via local fluorescence in-situ hybridization/karyotyping. Median PFS was 36.4 months with D-VMP versus 19.3 months with VMP after a median follow-up of 40.08 months (HR, 0.42; 95% confidence interval [CI], 0.34-0.51; P <0.0001; Figure A). Median PFS2 was not reached with D-VMP versus 42.3 months with VMP (HR, 0.55; 95% CI, 0.43-0.71; P <0.0001). The estimated 36-month OS rate was 78% with D-VMP versus 68% with VMP, with a significant benefit for OS observed for D-VMP versus VMP alone (HR, 0.60; 95% CI, 0.46-0.80; P = 0.0003; Figure B); median OS was not reached in either group and follow-up is ongoing. Additional efficacy data, including MRD negativity, and updated safety data will be presented at the meeting.
Conclusions: For the first time, we demonstrate that the addition of DARA to VMP prolongs OS in patients with transplant-ineligible NDMM, with a 40% reduction in the risk of death versus VMP alone after a median follow-up of 40 months. D-VMP continued to demonstrate a significant PFS benefit, which was also maintained during the subsequent line of therapy. These findings, together with the phase 3 MAIA study (DARA plus lenalidomide/dexamethasone vs lenalidomide/dexamethasone), continue to support the addition of DARA to frontline treatment regimens in patients with transplant-ineligible NDMM.
Daratumumab, Carfilzomib, Lenalidomide and Dexamethasone (Dara-KRd) Induction, Autologous Transplantation and Post-Transplant, Response-Adapted, Measurable Residual Disease (MRD)-Based Dara-Krd Consolidation in Patients with Newly Diagnosed Multiple Myeloma (NDMM)
- Abstract
- Presentation
Author(s): Luciano J. Costa, MD PhD, Saurabh Chhabra, MD, Kelly N. Godby, MD, Eva Medvedova, MD, Robert F. Cornell, MD MS, Aric C. Hall, MD, Rebecca W. Silbermann, MD, Racquel Innis-Shelton, MD, Binod Dhakal, MBBS, Diego DeIdiaquez, MD, Pamela Hardwick, RN, Yelak Biru, James L. Omel, MD, Parameswaran Hari, MBBS, MD, Natalie Scott Callander, MDLuciano J. Costa, MD PhD, Saurabh Chhabra, MD, Kelly N. Godby, MD, Eva Medvedova, MD, Robert F. Cornell, MD MS, Aric C. Hall, MD, Rebecca W. Silbermann, MD, Racquel Innis-Shelton, MD, Binod Dhakal, MBBS, Diego DeIdiaquez, MD, Pamela Hardwick, RN, Yelak Biru, James L. Omel, MD, Parameswaran Hari, MBBS, MD, Natalie Scott Callander, MD
Disclosures: Costa:Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Speakers Bureau; GSK: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy; Karyopharm: Consultancy; Fujimoto Pharmaceutical Corporation Japan: Other: Advisor. Cornell:Takeda: Consultancy; KaryoPharm: Consultancy. Silbermann:Janssen, Sanofi: Other: Consultant/Advisor. Dhakal:Amgen: Membership on an entity’s Board of Directors or advisory committees, Research Funding; Celgene: Honoraria; Takeda: Membership on an entity’s Board of Directors or advisory committees; Janssen: Membership on an entity’s Board of Directors or advisory committees; Sanofi: Membership on an entity’s Board of Directors or advisory committees. Omel:Celgene, Takeda, Janssen: Other: Patient Advisory Committees. Hari:Kite: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; BMS: Consultancy, Research Funding; AbbVie: Consultancy, Honoraria; Cell Vault: Equity Ownership; Sanofi: Honoraria, Research Funding; Spectrum: Consultancy, Research Funding; Amgen: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding.
OffLabel Disclosure: Carfilzomib for newly diagnosed multiple myeloma
Background: The CD38-targeting antibody daratumumab, when combined with a proteasome inhibitor or with an immunomodulatory agent (IMiD) increases depth and duration of response in multiple myeloma (MM). Depth of remission post initial therapy as assessed by MRD predicts long term outcome in NDMM. We hypothesized that the combination of daratumumab, carfilzomib, lenalidomide and dexamethasone (Dara-KRd) would be safe and highly active in patients with NDMM. In addition, we assessed the feasibility of using MRD by next generation sequencing (clonoSEQ® method, sensitivity 10-6) to inform the use and duration of post-transplant Dara-KRd consolidation.
Methods: Eligible patients (pts) had NDMM requiring treatment, creatinine clearance >40 ml/min, adequate liver and cardiac function, ECOG performance status 0-2 with no age limit. Treatment cycles consisted of daratumumab 16 mg/kg IV days 1,8,15,22 (with typical reduction in frequency with subsequent cycles), carfilzomib 56 mg/m2 IV days 1,8,15, lenalidomide 25 mg PO days 1-21 and dexamethasone 40 mg PO/IV days 1,8,15,22 repeated every 28 days. Patients received 4 cycles of Dara-KRd as induction, autologous transplantation, and received 0, 4 or 8 cycles of Dara-KRd consolidation, according to MRD status at each phase of therapy. MRD was evaluated by clonoSEQ® (NGS-MRD; Adaptive Biotechnologies, Seattle, WA) at end of induction, post-transplant, and during each 4-cycle block of Dara-KRd consolidation. Primary endpoint was achievement of MRD negative remission (<10-5) as defined by IMWG consensus. Secondary endpoints included MRD <10-6, complete response (CR) by IMWG criteria at end of induction and upon completion of consolidation, and rate of imaging (assessed by PET/CT scan) plus MRD-negative CR. Patients received therapy until achievement of two consecutive MRD reads <10-5 (confirmed MRD-negative remission; e.g., post-induction and post-transplant or post-transplant and during consolidation). Confirmed MRD-negative pts received no further therapy and were observed with surveillance for MRD resurgence 6 and 18 months after treatment discontinuation. Patients completing consolidation without confirmed MRD-negative remission received standard lenalidomide maintenance (NCT03224507).
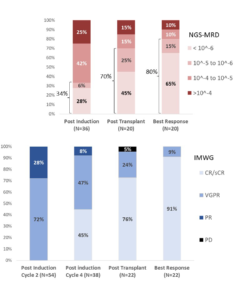
Results: Currently 69 pts have been enrolled, 38 have completed induction and 22 have completed post-transplant assessment. Median age was 61 (range 38-79) years, 13 (19%) had ISS 3, and 20 (29%) had high-risk chromosomal abnormalities [del17p, t(4;14) or t(14;16)]. Sixty-six (96%) pts had MRD trackable by clonoSEQ® and 100% of the expected MRD datapoints were successfully obtained. All patients responded by end of induction cycle 2, 92% of pts obtained VGPR or better after induction and 91% of patients who have reached transplant obtained CR/sCR as best response on therapy (Figure). MRD-negative remission (<10-5) rate was 34%, 70% and 80% after induction, transplant and at best response, respectively (Figure). Rates of MRD <10-6 were 28%, 45% and 65% respectively. No patient discontinued therapy due to toxicity. One patient died from metapneumovirus pneumonia post-transplant, considered not related to investigational agents. Most common grade 3 and 4 AEs were neutropenia (n=7), infection (n=6), insomnia (n=4), hyperglycemia (n=2) and rash (n=2). There were 15 serious AEs including pneumonia (n=5), fever and neutropenia (n=2), pulmonary embolism (n=1), and atypical hemolytic uremic syndrome (n=1). All 11 patients who have achieved confirmed MRD-negative remission and discontinued therapy also achieved imaging plus MRD-negative CR and none had relapse or resurgence of MRD with short follow up (0.8-7.3 months). Longer follow-up, post-induction and post-transplant MRD assessment for at least 69 and 41 pts, respectively, will be presented at the meeting.
Conclusion: This is the first report of monoclonal antibody-based quadruplet regimen with MRD-based response-adapted therapy in NDMM. Dara-KRd induction, autologous transplant and Dara-KRd consolidation guided by MRD is feasible, safe and leads to high proportion of patients achieving CR/sCR, IMWG MRD-negative CR, imaging plus MRD-negative CR and MRD <10-6. This approach can form the basis for clinical efforts to reduce the burden of continuous therapy in those with confirmed MRD-negative remissions.
Efficacy and Safety of Carfilzomib at 56mg/m2 with Cyclophosphamide and Dexamethasone (K56Cd) in Newly Diagnosed Multiple Myeloma Patients Followed By ASCT or K56Cd Consolidation: Initial Results of the Phase 2 Cardamon Study
- Abstract
- Presentation
Author(s): Kwee Yong, Rakesh Popat, William Wilson, Gavin Pang, Richard Jenner, Ruth M De Tute, BSc,MSc, Karthik Ramasamy, MBBS MRCP FRCPath PhD, Matthew Streetly, MD, Jamie Cavenagh, MD PhD, Jonathan Sive, MD, Michael Chapman, PhD MRCP, FRCPath, Ceri Bygrave, MD FRCPath, MPhil, Beth Phillips, MBBS, FRCPath, BSc, Selina J Chavda, Andres E. Virchis, FRCP, FRCPath, Reuben Benjamin, FRCPath, MRCP, MBBS, PhD, Sarah Arnott, Fenella Willis, Sandra Hassan, MBBS, MRCP, FRCPath, MDRes), Sally Moore, Laura Clifton-Hadley, PhDBSc, Roger G Owen, MD
Disclosures: Yong:Sanofi: Speakers Bureau; Amgen: Research Funding, Speakers Bureau; Autolus: Consultancy; Janssen: Speakers Bureau; Takeda: Research Funding, Speakers Bureau. Popat:Celgene Corporation: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees, Other: travel, accommodations, expenses; Janssen: Honoraria, Other: travel support to meetings; AbbVie: Consultancy, Membership on an entity’s Board of Directors or advisory committees; GSK: Consultancy, Honoraria; Takeda: Honoraria, Other: travel, accommodations, expenses. Ramasamy:Takeda: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity’s Board of Directors or advisory committees, Research Funding; NAPP Pharmaceuticals Ltd.: Research Funding; Janssen-Cilag Ltd.: Research Funding; Oncopeptides and Sanofi: Honoraria, Membership on an entity’s Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity’s Board of Directors or advisory committees. Chapman:Takeda: Honoraria. Benjamin:Allogene: Research Funding; Gilead: Honoraria; Novartis: Honoraria; Pfizer: Research Funding; Amgen: Honoraria; Takeda: Honoraria; Servier: Research Funding; Eusapharm: Consultancy. Owen:Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Travel/ meeting support.
OffLabel Disclosure: Carfilzomib is used with cyclophosphamide as 1st line treatment for myeloma
Introduction: Carfilzomib (20/36mg/m2) triplets with Lenalidomide-Dexamethasone (KRd), or Cyclophosphamide-Dexamethasone (KCd) are safe and effective in patients with newly diagnosed multiple myeloma(NDMM). The higher dose of 56mg/m2 is effective as a doublet with Dexamethasone in the relapsed setting, but there is limited data on this dose in triplet combinations in the frontline setting.
Aim: The CARDAMON study evaluated KCd with bi-weekly carfilzomib (56mg/m2) as induction in NDMM patients, and the benefit of ASCT versus K56Cd consolidation followed by carfilzomib maintenance. Co-primary endpoints were major response (≥VGPR rate) to 4 induction cycles of K56Cd, and 2-year PFS for ASCT versus K56Cd consolidation. Here we report interim analysis of the first primary endpoint of ≥VGPR rate to K56Cd induction.
Methods: Transplant eligible ND patients received 4 x 28d cycles of K56Cd (carfilzomib:20/56mg/m2, IV d1, 2, 8, 9, 15, 16, cyclophosphamide 500mg orally d1, 8, 15 and dexamethasone 20mg d1, 2, 8, 9, 15, 16). Responding patients with a successful stem cell harvest (PBSCH) were randomised to autologous stem cell transplant (ASCT) or 4 more cycles of K56Cd as consolidation, followed by 18 months carfilzomib maintenance (K56 days 1, 8, 15) for both arms. Trial recruitment completed in July 2019. Response was assessed by IMWG criteria; all patients had MRD testing by multi-parameter flow cytometry (10-5) after PBSCH. Adverse risk genetics was any one of t(4;14), t(14;16), t(14,20) or del(17p).
Results: 281 pts were registered between 06/2015 and 07/2019; we report outcomes for 252 patients who either completed induction or came off study before end of induction. Median age was 58yrs(33-74), 91% ECOG 0-1, 45.2% ISS I, 24.7% adverse risk (48.5% when including 1p/1q+). Best response at end of induction or after PBSCH (n=250) was: ≥VGPR 59.2%, ORR 87.6%. ≥VGPR rate in adverse risk patients was 53.4% vs 61.9% in standard risk(SR), (p=0.25), ORR was similar: adverse risk, 87.9% vs standard risk, 88.1%. Post-PBSCH, 24.1% of patients were MRD-negative (patients who were withdrawn due to insufficient induction response or toxicity and those with an inconclusive result were grouped with the MRD-positive). Of 19 patients in sCR/CR, 9 were MRD-negative(47.4%) while 40/110 (36.4%) of VGPR patients were MRD-negative. MRD-negative rates in adverse and standard risk patients were 22.8% and 24.7% respectively. 10 patients progressed during or at end of induction, and 12 were withdrawn for toxicity.
There were 4 deaths during induction, one from myocardial infarction, the other 3 from cardiac arrest, associated with bronchopneumonia and sepsis. During induction, 114 serious adverse events (SAEs) were reported in 72/252 patients, notable ones were thrombotic microangiopathy (2), grade 3 cardiac ischaemia (4), infection (16.3%, mainly lung), renal impairment (6), G3 hypertension (3), thromboembolism(2). Specific guidance for hypertension management was incorporated. 25% of patients are currently reported to have received a dose modification during induction. Full details of adverse events and dose intensity will be presented at the meeting.
Conclusion: K56Cd is an effective induction regimen in NDMM patients, and has equivalent MRD negative rates in adverse and standard risk disease. The SAE profile is in keeping with published safety data with carfilzomib.
Disclaimer

© 2020 American Society of Hematology. All rights reserved. ASH® and the ASH logo are registered trademarks of the American Society of Hematology. Used with permission.
This content is provided for informational purposes and personal use only, and is not intended to provide medical advice, diagnosis, or treatment or for commercial use. The ideas and opinions expressed herein do not necessarily reflect those of the American Society of Hematology (ASH®). The mention of any product, service or therapy in this collection of materials should not be construed as an endorsement of the products mentioned.
Copyright © 2020 Springer Healthcare Limited. Part of the Springer Nature Group.